ProJect Pharmaceutics is glad to announce its participation as exhibitor at the upcoming CPhI trade fair, taking place in Madrid, Spain, October 10-13, 2018. We look forward to welcoming you at our booth #14.1 D 92.
News
ProJect Pharmaceutics enters into collaboration with business partners in Asia
ProJect Pharmaceutics is delighted to expand the global presence of its service portfolio towards the Asian market. Entering into a collaboration with business partner BiGEN in South Korea, ProJect Pharmaceutics becomes a cross-country competence hub for formulation and process development.
On top, ProJect Pharmaceutics can now offer its clients to act as a gate opener to the Asian market with regard to drug substance manufacturing as well as drug product commercialization.
Meet ProJect Pharmaceutics at BIO 2018
ProJect Pharmaceutics is glad to announce its participation as exhibitor at the upcoming BIO International Convention trade fair, taking place in Boston, U.S.A., June 4-7, 2018. We look forward to welcoming you at our booth #1443.
Formulation and lyophilization process development for live virus vaccines
ProJect Pharmaceutics expands its service portfolio to meet customer’s increasing need for the development of GMO S2 and BSL-2 biological products including live viruses.
ProJect Pharmaceutics is one of the leading European service providers offering tailored pharmaceutical formulations and lyophilisation processes for therapeutic proteins, peptides and other therapeutic compounds.
New safety facilities in dedicated labs and a deep understanding of the challenges when processing virus vaccines enable ProJect Pharmaceutics to provide specific solutions for this group of products of high complexity.
Smart formulations and manufacturing processes to safeguard the efficacy of the finished drug product are provided together with mandatory safety requirements up to GMO S2 and BSL-2 classification.
Maintaining the native quaternary structure of the virus capsid and avoiding virus aggregation is the key to maintain its full biological activity even under stress conditions like elevated temperature, freezing and freeze drying. Cutting-edge analytical high-throughput methods have been implemented in our biological safety lab in order to select the most effective combination of excipients for the final drug product. The GMO S2 and BSL-2 safety lab is equipped with a dedicated pilot freeze dryer to design tailored lyophilisation cycles both maximally robust and efficient. PJP’s long lasting experience and comprehensive know-how of lyophilisation enables our customers to follow new paths in process technology. Customers will benefit from safer products with an optimized stability profile and reduced manufacturing costs.
For more information please visit: www.project-pharmaceutics.com or contact:
ProJect Pharmaceutics GmbH
Fraunhoferstraße 22
D-82152 Martinsried
+49 (0) 89 452289700
ProJect Pharmaceutics enters collaboration with German CMO as the preferred development partner for lyophilization
ProJect Pharmaceutics is a contract research and development organization (CRO) specialized in formulation and process development for parenteral drug products. Adjacent to its service portfolio, ProJect Pharmaceutics operates a broad network towards contract manufacturing organizations (CMOs) for drug substance and drug product to support its clients towards selecting the best fit for their products’ manufacturing requirements.
As state-of-the-art development experts ProJect Pharmaceutics is teamed up with state-of-the-art manufacturing experts at LYOCONTRACT GmbH to provide high-quality parenterals from pre-clinical to clinical and large commercial scale.
ProJect Pharmaceutics’ clients can profit from a direct line to a) liquid fill & finish services on a modern filling line, and b) manufacturing of lyophilized products for clinic and market supply on high-class commercial lyophilization plants.
The CMO’s clients can profit from a direct line to lyophilization cycle development and/or optimization towards a robust but efficient lyo cycle design – an efficient lyo cycle can not only safe our clients time and thus pure money on manufacturing batches, but also facilitate to get a manufacturing slot way easier at CMOs by not blocking the manufacturing line for days or weeks
Join ProJect Pharmaceutics at the upcoming World ADC
ProJect Pharmaceutics is glad to announce its participation as exhibitor at the upcoming World ADC Conference, taking place in Berlin, Germany, February 21-23, 2017. The conference will bring together international experts on ADCs from industries, universities and CROs.
High Protein Concentration Formulations
Munich, October 29, 2015 – ProJect Pharmaceutics introduces a science-based approach to develop best-in-class formulations for convenient use in pre-filled syringes
Transforming therapeutic proteins into high-concentrated, stable and easy to use medications for subcutaneous self-injection can add up to a major challenge along their clinical development. A formulation’s fundamental qualifications such as appropriate physico-chemical stability, container material compatibility and viscosity must be taken into account to support an acceptable in-syringe shelf-life as well as convenience in regard to syringe-ability. However, in order to take all of the requirements to a maximum and develop a pioneering new drug in a pre-filled syringe presentation, a great extent of knowledge, expertise and sophisticated analytics is needed that are sparingly found in this developing market to date.
ProJect Pharmaceutics, one of the experts in formulation and process development for parenteral drugs, has specialized in this emerging field with a key expertise in development of stable, effective and syringe-able formulations and their eligible processes. Be it conformation of de novo drug products towards a pre-filled syringe application or transfer of existing drug products into the like, ProJect Pharmaceutics features all the required key qualifications in that field.
By applying an elaborate science-based approach to meet well-discussed critical quality attributes such as e.g. maximum effectiveness, high concentration for low injection volume, low aggregate content and low viscosity, ProJect Pharmaceutics offers profound guidance and support on the development of pioneering parenteral biologics.
‘Predictive formulation analytics’ positions ProJect Pharmaceutics towards a rational design of high-concentrated but low viscous and easy injectable formulations:
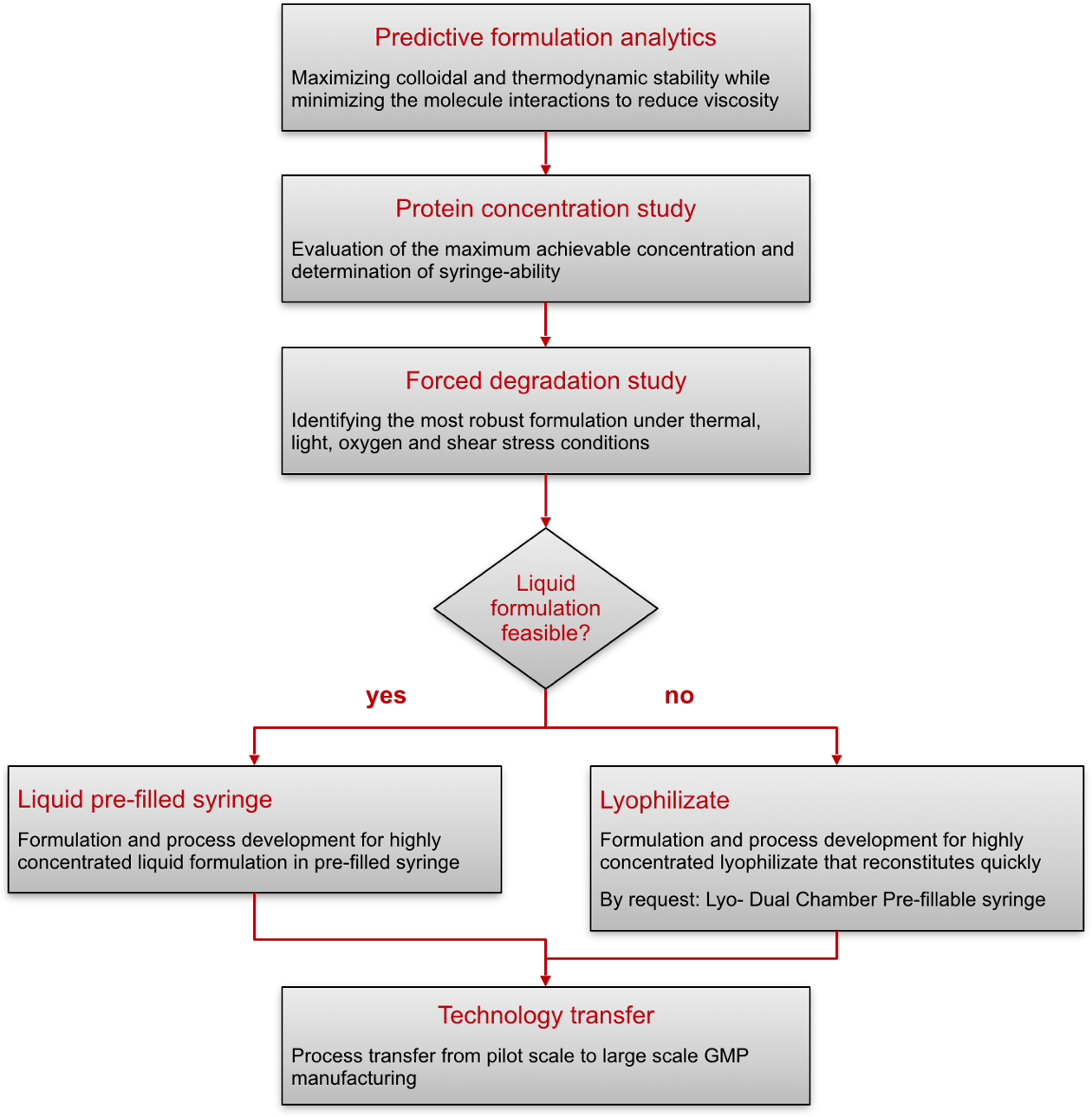
Minimizing viscosity and aggregation by actively maximizing repulsive protein-protein interaction in solution is one of the key techniques used. Additionally, shifting thermodynamic protein stability in solution to its maximum prove another key requisite promoting long-term stability of liquid formulations in pre-filled syringes.
Designed formulations’ syringe-ability is measured directly in-system mimicking the typical administration procedure of the patient instead of through viscosity only.
In case stability in aqueous solution is found to be limited by the biologic ́s inherent sensitivity freeze-drying clears the way forward. Innovative design of lyo-formulations and freeze-drying processes developed by ProJect Pharmaceutics reliably overcome the hurdles of reconstituting high-concentrated protein lyophilizates.
Supply of aseptic pre-clinical study material in pre-filled syringes at pilot scale complete ProJect Pharmaceutics’ excellence as your pharmaceutical service provider in this exigent field of highly concentrated protein formulation development.
Meet ProJect Pharmaceutics at Bio Korea 2015
Join Dr. Andreas Schütz for his presentation: “Formulation and lyophilization process development for biopharmaceutics” at Bio Korea 2015 from April 8–10, in Seoul, COEX international convention center. His talk is scheduled April 8th, 15:30 at Business/Technology Presentation Forum.
Please contact our office at if you need further details on the conference or wish to meet us at there.
Join Project Pharmaceutics at the upcoming World ADC
Project Pharmaceutics is glad to announce its participation as exhibitor at the upcoming World ADC Conference, taking place in Frankfurt, Germany, February 17-20, 2014. This conference will bring together international experts on ADCs from industries, universities and CROs.
ProJect Pharmaceutics and TUM-Spin-off ImevaX announce agreement on development of pharmaceutical formulation for IMX101
Munich/Martinsried, November, 2013
ProJect Pharmaceutics announces that it has signed a development agreement with ImevaX, a spin-off project from the research group of Prof. Dr. Markus Gerhard from the Technische Universität München, Institute for Medicinal Microbiology, Immunology and Hygiene, aimed at developing a formulation for a recombinant vaccine against Helicobacter pylori. IMX101 is a multicomponent vaccine, making the formulation development particularly challenging.
The contract covers activities in developing a rational formulation based on ProJect Pharmaceutics´ Predictive Formulation Analytics. This technology offers an innovative scientific approach for designing optimized protein formulations by determining the most favorable composition for the native structure of the protein with regard to its intra- and intermolecular physicochemical properties. By analyzing the response to certain excipients the most promising formulation candidates can be identified quickly and reliably reducing the need for extensive stability testing.
Promising formulation candidates have successfully been evaluated and are continuously optimized within the development program since its launch in mid 2013. Financial terms of the current agreement are not disclosed.
About project ImevaX
As part of the GO-Bio start up program of the German Ministry of Research and Education (BMBF), the TUM-based Spin-off project ImevaX from the research group of Prof. Dr. Markus Gerhard from the Technische Universität München, Institue for Medicinal Microbiology, Immunology and Hygiene, develops highly specific vaccines against pathogens that cause chronic infectious diseases. Its technology identifies immune modulatory bacterial factors that are then used to develop effective vaccines against infections of global relevance. ImevaX is planned to be founded as a biotech company in 2014.
For more information please visit: www.imevax.com or contact:
Medizinische Mikrobiologie, Immunologie und Hygiene
Trogerstraße 30
D-81675 München
+49 (0) 89 4140 2477
Prof. Dr. Markus Gerhard:
